
January 22, 2025
Cannabis produces a complex array of volatile organic compounds (VOCs) that contribute to its distinct aroma. These compounds include terpenes, volatile sulfur compounds (VSCs), and various non-terpenoid volatile compounds. This review provides an overview of the synthesis, distribution, and sensory perceptions of these major contributors to cannabis odor, beginning with the fundamental role of isoprene as the building block for terpenes.
Synthesis of Terpenes: Isoprene as the Building Block
Terpenes are a large and diverse class of organic compounds found in all living organisms. Five-carbon isoprene (C₅H₈) units serves as the fundamental building block for all terpenes, which are constructed by the sequential addition of these units. The general formula for terpenes is (C5H8)n, where “n” represents the number of isoprene units. The immense diversity of terpenes arises from the various ways bonds form and twist between isoprene units.
Terpenes are synthesized through two primary pathways in plants: 1) Methylerythritol Phosphate (MEP) Pathway and 2) Mevalonic Acid (MVA) Pathway. Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) serve as key intermediates in both pathways, leading to the formation of geranyl diphosphate (GPP) and farnesyl diphosphate (FPP), which are precursors for monoterpenes and sesquiterpenes, respectively. GPP is also a precursor to cannabigerol acid (CBGA), making the cannabinoid and terpene synthesis pathways intimately linked (Figure 1).
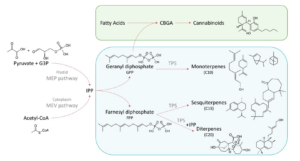
Monoterpenes and Sesquiterpenes
Monoterpenes are synthesized via the MEP pathway, where two isoprene units combine to form GPP. Key monoterpenes in cannabis include limonene, myrcene, and pinene. Limonene offers citrus-like notes and is known for its fresh, uplifting aroma[1]. Myrcene imparts a musky, earthy smell and is one of the most abundant terpenes in cannabis[2]. Pinene, found in many plant species, has a distinctive pine-like aroma[3]. Monoterpene synthesis dominates the early growth stages and becomes most concentrated during the flowering phase[4].
Sesquiterpenes are synthesized via the MVA pathway, where three isoprene units combine to form FPP. Important sesquiterpenes in cannabis include β-caryophyllene and humulene. β-Caryophyllene is responsible for a peppery aroma and interacts with the CB₂ receptor, exhibiting anti-inflammatory properties[5]. Humulene contributes to woody and earthy aromas and is also found in hops[6]. Sesquiterpene synthesis typically occurs later in the plant’s development compared to monoterpenes, peaking during the flowering stage and influencing the plant’s spicy or woody scent[7].
The biosynthesis of terpenes is controlled by a family of enzymes known as terpene synthases (TPS), which catalyze the cyclization and modification of GPP and FPP to produce the diverse array of terpenes found in cannabis[8]. Recent studies have demonstrated that genetic variation in TPS genes is associated with the chemical diversity observed in cannabis[9]. Vergara et al. (2021) found that this genetic variation influences terpene profiles, affecting strain classification and consumer perception.
Volatile Sulfur Compounds (VSCs)
Volatile sulfur compounds have gained attention for their significant role in the “skunky” aroma often associated with cannabis[10](Figure 2). These compounds are synthesized through metabolic pathways involving sulfur-containing precursors. Key VSCs include 3-methyl-2-butene-1-thiol, a highly pungent compound also found in garlic and hops[11], and prenylated thiols that intensify during the curing process[12].
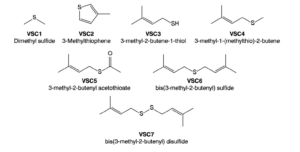
VSCs are produced primarily in the later stages of cannabis flowering and peak during curing[13]. Advancements in analytical techniques have enhanced the detection and quantification of these compounds, improving our understanding of their role in cannabis aroma[12]. Comprehensive two dimensional gas chromatography has been particularly instrumental in identifying new families of prenylated VSCs, offering deeper insights into their contributions to the overall scent profile[10].
Sensory Perception of Cannabis Volatiles
The complex interplay among volatile compounds produced by cannabis drives the perception of the user. Monoterpenes such as limonene and pinene provide fresh, citrusy, or pine-like aromas. These lighter molecules contribute bright and uplifting notes to the fragrance profile. In contrast, sesquiterpenes like β caryophyllene introduce deeper, more robust aromas, offering spicy, woody, or peppery notes that add complexity and warmth to the sensory experience[7]. Figure 3 summarizes aroma descriptors reported by a sensory panel[13].
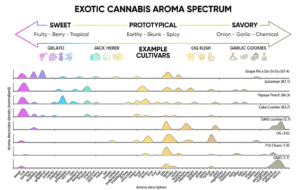
VSCs, particularly 3-methyl-2-butene-1-thiol, are associated with skunky, pungent odors[10]. Even in vanishingly low concentrations, these compounds can dominate the scent profile, contributing to the overall pungency. Non-terpene compounds like alcohols, aldehydes, and esters add earthy or floral nuances[13]. The balance and concentration of these compounds within each chemovar contribute to the unique aromas and flavors of different cannabis strains[14]. Sensory evaluation studies have demonstrated strong correlations between chemical profiles and human perception, providing valuable insights for breeders and producers. By understanding how these compounds interact to shape aroma and flavor, the industry can develop cannabis products with desired aromatic profiles, enhancing the overall consumer experience[15].
Conclusion
The aroma of cannabis is shaped by a wide range of volatile compounds. Monoterpenes and sesquiterpenes, derived from isoprene building blocks, dominate the terpenoid profile, while volatile sulfur compounds and non-terpenoid volatiles add complexity to the scent. Genetic variation in terpene synthase genes plays a crucial role in terpene diversity, influencing strain classification and consumer perception.
References
- Chacon, F. T., Raup-Konsavage, W. M., Vrana, K. E., & Kellogg, J. J. (2022). Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines, 10(12), 3142.
- Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., & Jantrawut, P. (2020). The Cannabis Terpenes. Molecules, 25(24), 5792.
- Tholl, D. (2015). Biosynthesis and Biological Functions of Terpenoids in Plants. Advances in Biochemical Engineering/Biotechnology, 148, 63–106.
- Booth, J. K., & Bohlmann, J. (2019). Terpene synthases and terpene variation in Cannabis sativa. Plant Molecular Biology, 100(6), 633–644.
- Gertsch, J., Leonti, M., Raduner, S., et al. (2008). Beta-caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences, 105(26), 9099–9104.
- Pate, D. W. (1994). Chemical ecology of cannabis. Journal of the International Hemp Association, 2(2), 29–32.
- Tholl, D., Chen, F., Petri, J., Gershenzon, J., & Pichersky, E. (2005). Two Sesquiterpene Synthases from Arabidopsis thaliana Catalyze the Formation of Cadinene and Eremophilene. Archives of Biochemistry and Biophysics, 448(1–2), 146–156.
- Booth, J. K., Page, J. E., & Bohlmann, J. (2017). Terpene synthases from Cannabis sativa. PLoS ONE, 12(3), e0173911.
- Vergara, D., et al. (2021). Cannabis labelling is associated with genetic variation in terpene synthase genes. Nature Plants, 7(11), 1330–1334.
- Oswald, I. W. H., et al. (2021). Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega, 6(48), 31667–31676.
- Rice, S., Koziel, J. A., & Dharmadhikari, M. (2020). Characterization of the key aroma compounds in cannabis smoke. Journal of Chromatography A, 1629, 461502.
- Oswald, I. W. H., et al. (2020). Profiling of Volatile Sulfur Compounds in Cannabis Using Comprehensive Two-Dimensional Gas Chromatography. Journal of Natural Products, 83(5), 1428–1438.
- Oswald, I. W. H., et al. (2023). Minor, Nonterpenoid Volatile Compounds Drive the Aroma Differences of Exotic Cannabis. ACS Omega, 8(36), 39203–39216.
- Elzinga, S., Fischedick, J., Podkolinski, R., Raber, J. C., & Erkelens, T. (2015). Distinguishing Cannabis sativa and Cannabis indica Strains Based on Terpene and Cannabinoid Composition. Cannabis and Cannabinoid Research, 1(1), 1–7.
- Russo, E. B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364.




